How do you begin to understand an organ as complex as the brain? Arguably the most intricate system in living organisms, the brain is composed of millions to billions of cells of many different types – not just neuronal cells (the nerves that are what most people consider to be “brain cells”), but also a vast array of non-neuronal cells that support brain function, such as vascular and immune cells. Over the past decade, significant progress has been made in identifying and classifying the different cell types found in the brain using techniques such as high-throughput single-cell sequencing, which has revealed important information on the diversity of cell types and key gene expression profiles.
Cells in the brain consist of multiple different subclasses, each with a distinct purpose and function, including excitatory and inhibitory neurons. Importantly, many of these cell types are specific to different brain regions.
The layers of detail don’t end there. Not only are there a vast array of different cell types to characterize and understand, they are also arranged in highly specialized and intricately integrated circuits that range in scope from small-scale cell neighborhoods all the way up to higher brain structures such as the amygdala and hypothalamus. Therefore, to develop a comprehensive detailed mouse brain map, spatial context and analysis of cell-to-cell interactions are critical.
In a new study – currently publicly available as a pre-print – a group of scientists pooled their efforts to create a detailed cell atlas of the mouse brain1. A necessarily enormous undertaking, the data presented in this paper are the result of a multi-center collaboration spanning the United States. Spearheaded by Hongkui Zeng, Director of the Allen Institute for Brain Science in Seattle, the paper lists over 70 authors from institutions such as Harvard, the University of Pennsylvania, and Genentech.
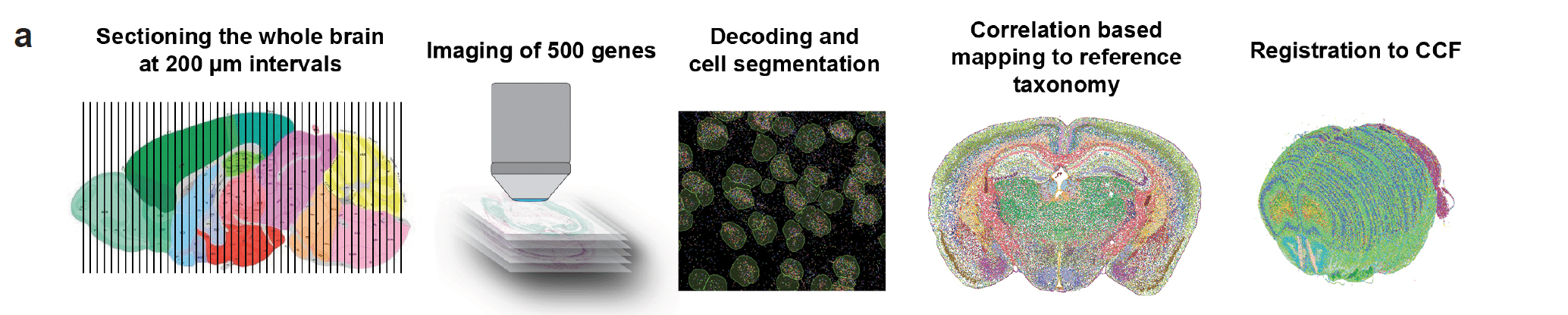
Figure 1: Workflow for generating MERFISH data with the MERSCOPE® Platform to create the mouse brain map.
Expression of up to 500 genes was assessed in over 4 million individual cells across multiple tissue sections that spanned the entire brain. Individual cell data was mapped back onto each tissue slice and ultimately the Allen Mouse Brain Common Coordinate Framework (CCF) to define brain regions in three-dimensional space. See preprint for full figure.
The researchers used multiple technologies to characterize brain cells, including Vizgen’s MERSCOPE® Platform which allowed them to accurately map each cell to a specific location within the 3D space of the whole mouse brain (figure 1). A total of 4.3 million cells were analyzed, using a panel of 500 genes per cell, by MERSCOPE for this study. The integration of spatial context allowed researchers to understand not just what the cells are but how they interact with each other and form these complex neural circuits and substructures within the brain.
One of the most striking finds with the new mouse brain nmap was the correlation between cell types and their spatial specificity – every subclass has a unique and specific spatial localization pattern within the brain. In addition to characterizing the cells and their precise spatial arrangement, researchers also looked at their behavior relative to their neighbors and were able to distinguish an extraordinary diversity of neurotransmitter expression patterns across cell types and brain areas, suggesting that they control specific modes of communication between cells.
The result of this extraordinary scientific collaboration is the first murine full brain atlas in three dimensions, and establishes a benchmark reference tool, revealing unique and previously undiscovered features of cell organization and communication in different brain regions (figure 2). This work represents a major milestone in our understanding of the makeup and functionality of the mammalian brain and will serve as a foundational resource to further understand how cell types interact to form neural circuits and what functional roles the different cell types play. It also sets the stage for building atlases across a lifespan, so we can better understand how the brain develops and grows. Additionally, it will be instrumental in the creation and analysis of cell atlases across species, including humans.
With access to the baseline cell characteristics and behaviors detailed in the atlas, researchers can investigate the dynamic changes that take place under different physiological conditions – health and disease – to identify specific processes, pathways, and cell types as markers for therapeutic outcomes, disease diagnosis and targets for novel treatments.

Figure 2: Neuronal cell type classification and distribution across the brain. UMAP representation (left) and representative MERFISH sections (right). See preprint for full figure.
Jiang He, Vizgen’s Scientific Cofounder and Senior Director of Scientific Affairs summed up what this research means to Vizgen and the field at large:
“This paper is a landmark study in the spatial genomics space as it establishes both an important benchmark reference and a foundational resource that will be built on by future researchers across multiple fields. We are incredibly proud that Vizgen’s technology has helped enable this research.”
The full mouse brain map preprint is available to read today! Access it here.
Reference:
- A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Yao et al. BIORXIV (2023). DOI: 10.1101/2023.03.06.531121.