
In a recent episode of Bio-IT World’s “Trends from the Trenches” podcast, Vizgen CEO Terry Lo sat down with BioTeam’s Stan Gloss to discuss the importance of spatial biology and how it has the potential to change healthcare’s future.
Listen to the full episode released on December 27, 2022: Vizgen’s Terry Lo on Creating Maps of Biology’s Backroads and check out highlights below:
“I’ve been lucky enough in my career where I’ve been involved with many different types of new technologies and breakthrough products, and this has got to be without a doubt the most exciting space that I’ve been involved with. It’s really been pretty amazing to see all of the enthusiasm and momentum that we’ve gained in the spatial biology field,” says Terry.
Spatial biology is the culmination of two different scientific approaches, resulting in the production of the most valuable information. He explains that on one hand, you have the histology side (or histopathology), which has historically been a very visual type of approach where you’re looking at macro-level types of information. In addition, you have the ability to look at genomic or molecular markers at an extremely high level (or high-plexity).
“The challenge has always been that these two have been very separate types of fields of science and technology, and now we have this ability to bring them together, and that’s really how you get spatial biology,” he says.
So how do we merge these two types of information into something meaningful?
“From a technology or informatics standpoint, a lot of what we’ve done in terms of the genomics information actually is very similar to what we would normally do with sequencing information, or with single-cell sequencing information. That sort of data is very similar to that type of data that we use today. What’s added now of course is the spatial dimensions and the spatial information that we have from imaging,” Terry explains.
We now have the ability to leverage both the histological and the molecular sides to produce high parameter data that can be built into what we are seeing visually. “We’ve never had a commercial platform that could take this information from a tissue and understand where the cells are and then understand what genes are being expressed within those cells in the tissue. That type of information has never been available commercially, so when we started Vizgen and launched the first platform in this space, this is the first time ever that now this data is accessible.”
When thinking of spatial biology, you can imagine a neighborhood with both good and bad neighbors. Equipped with this form of cell typing, you can not only determine at a high level which neighbors you may want to avoid, but you can also understand how a neighbor may act a certain way in one neighborhood and a different way in another. Terry explains that “just because it’s the same neighbor or the same character or the same cell, the way that they function and the way that they act can change dramatically depending on the environment that they’re in.”
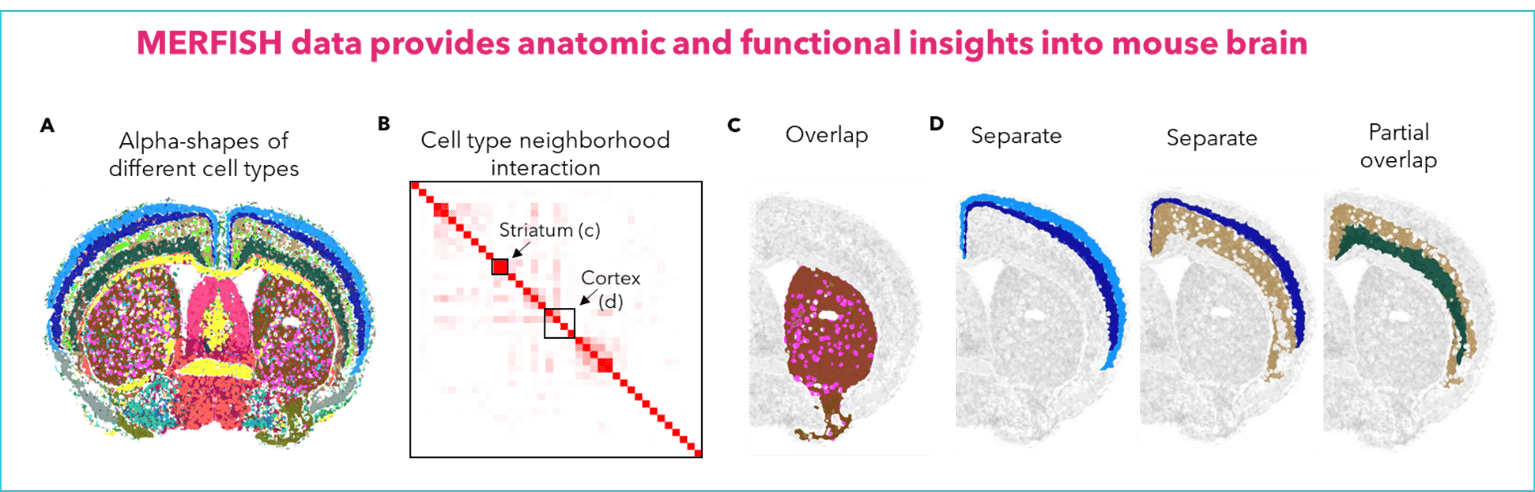
FIGURE 1: a) The alpha-shapes of different cell clusters in a mouse coronal section. b) Heatmap showing the cell cluster neighborhood overlap across cell clusters. The box regions representing MSN and cortical neuron clusters are shown in c and d. c) D1 and D2-MSNs are well mixed in striatum. d) Examples of different cortical neuron clusters showing either little (left and middle) or significant (right) overlap with other cortical neuron clusters.
The fundamental idea is that if you understand how a group of cells acts in different environments, you can map those behaviors and definitions in order to tease out some of the system’s biology.
One of the big questions is how spatial biology can change diagnostics. Since this type of technology focuses on tissue samples, it is more likely to be “front-end” in terms of discovery, target identification and confirming certain mechanisms of action in model organisms.
“It’s harder to apply this clinically in the neurosciences because typically we don’t take brain tissues from living patients. But if we switch over to different fields such as oncology where we are as standard taking biopsies or tissue samples, this absolutely is an area where we can certainly foresee this being used in a diagnostic-type setting, either as a prognostic tool, as a companion diagnostic or for using as a diagnosis method,” he explains.
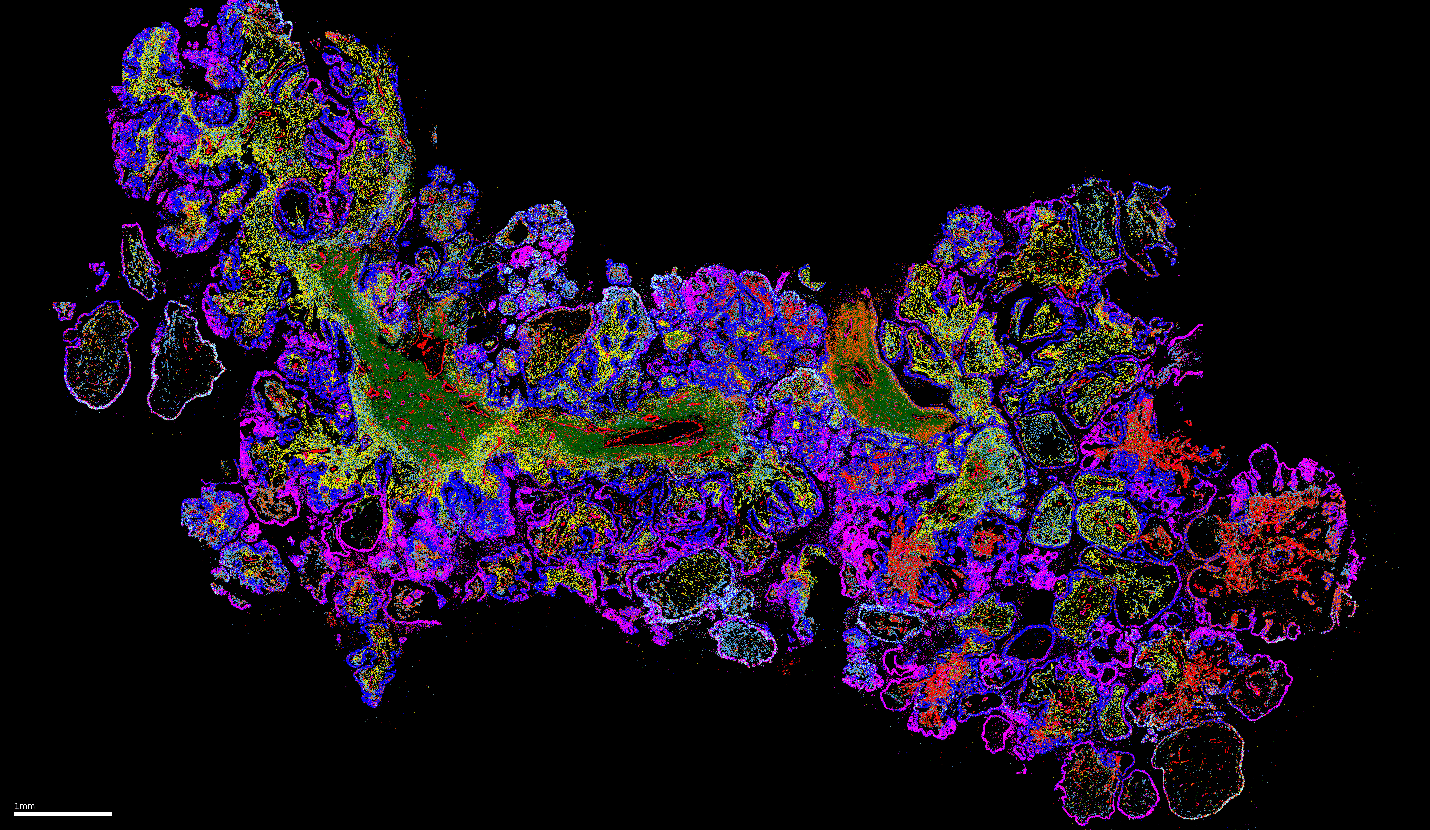
FIGURE 2: Spatial Distribution of Cell Types in Ovarian Cancer. During a MERSCOPE® Platform experiment, cell types across multiple cancers were spatially profiled using a 500 gene panel in combination with the cell boundary staining kit to label individual cells. This data image shows that MERSCOPE can precisely and brilliantly identify the relevant cell types across a wide variety of cancer types preserved in FFPE format, including ovarian cancer as displayed here.
So where do we go from here? According to Terry, we are at an early stage in terms of being able to bring this type of information to the research field. The first large step is to make this technology as widely adopted as possible, equipping scientists and researchers with the right types of data that they can leverage to advance their fields.
“My hope is that over the next 2-3 years what we’re going to see – and this is actually what we’re seeing today – is a widespread adoption and quick utilization of trying to access this data, and then looking for what these clinical applications or the clinical value is going to be downstream by using this scientific data,” concluded Terry.